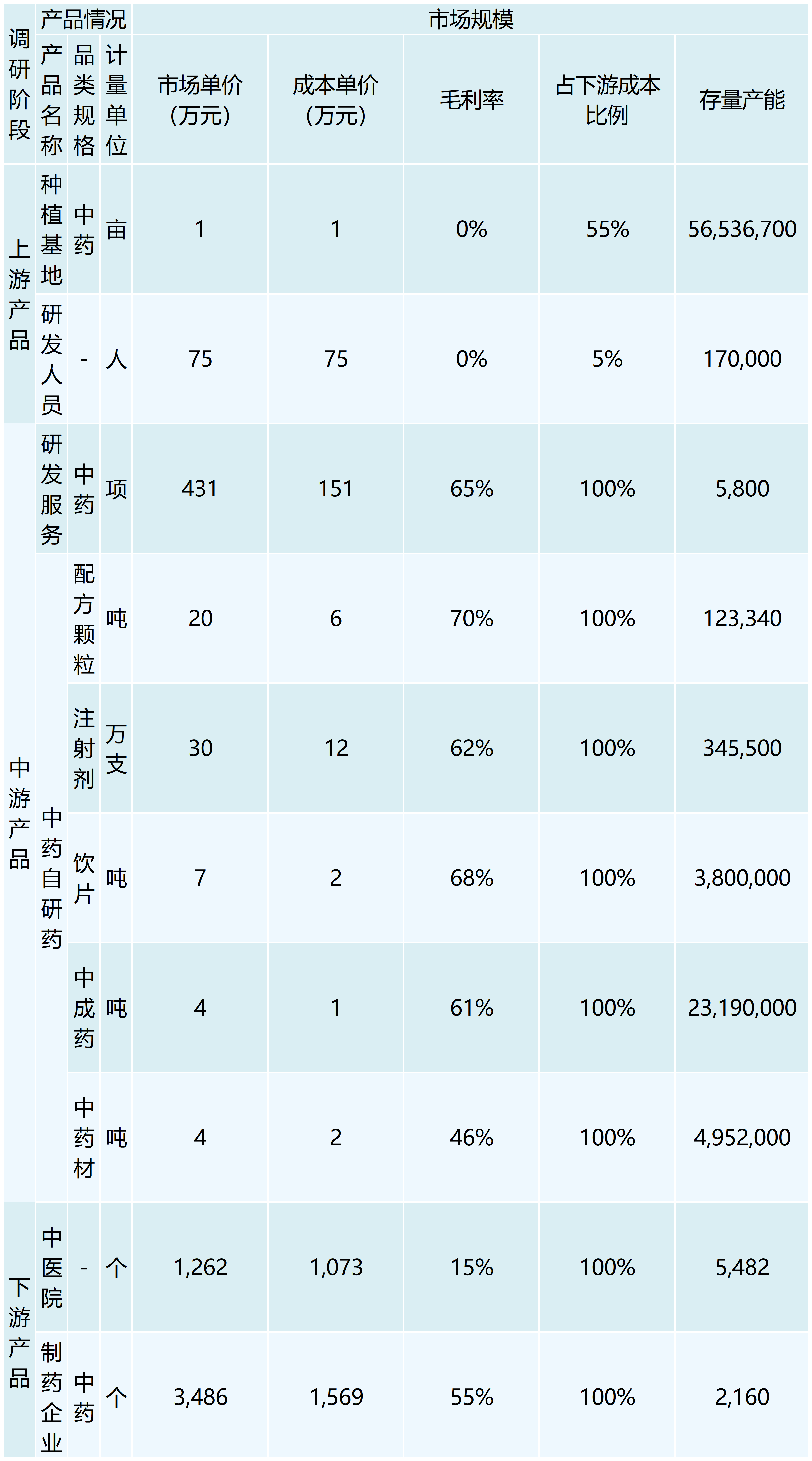
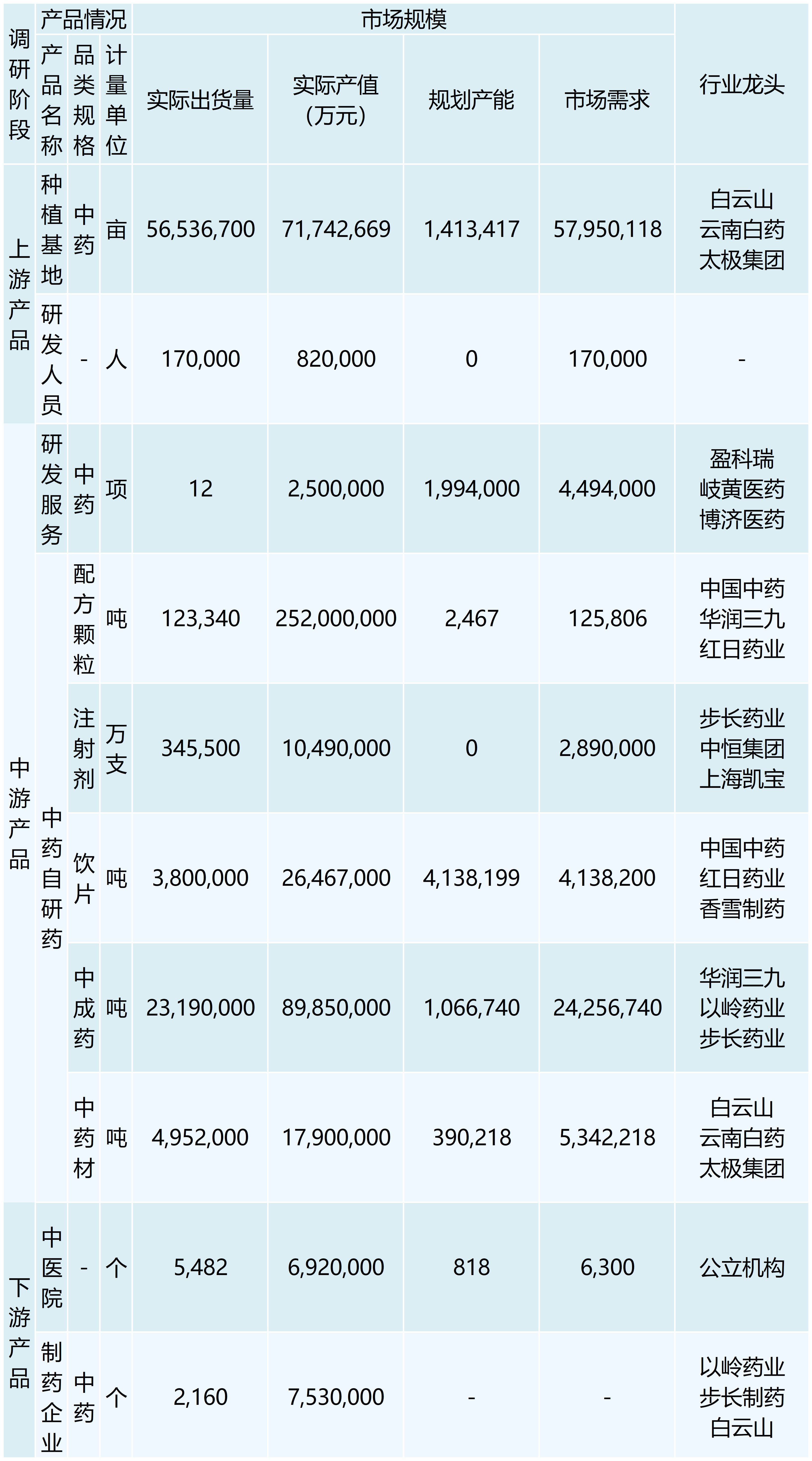
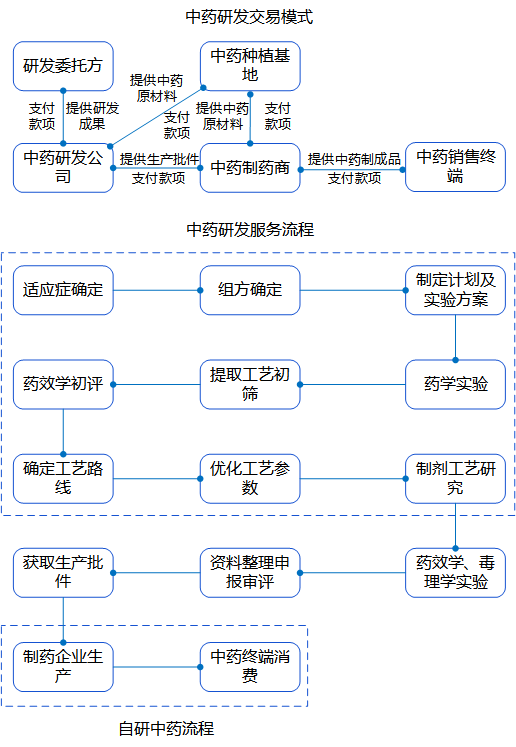
In recent years, the state has issued a number of important policies to encourage innovation in traditional Chinese medicine, driving the rapid development of the industry.2021 The General Office of the State Council issued “Several Policy Measures on Accelerating the Development of Chinese Medicine Features”, optimizing the review and approval of new Chinese medicine, which is conducive to the speed and time of approval and listing.2022 The Ministry of Industry and Information Technology and other nine committees issued the “14th Five-Year Plan for the Development of the Pharmaceutical Industry,” which indicated that it is necessary to increase the support for the scientific and technological innovation of traditional Chinese medicine. In 2022, the Ministry of Industry and Information Technology and other nine committees released the 14th Five-Year Plan for the Development of Pharmaceutical Industry, stating that they would increase support for scientific and technological innovation of Chinese medicine, and strengthen the research and development of new Chinese medicines based on ancient classic prescriptions, experienced prescriptions of famous Chinese medicine practitioners, and active ingredients or components, etc. In 2022, the size of the domestic CRO market is expected to be close to 90 billion yuan, and the size of the CRO market of traditional Chinese medicine is estimated to be 25 billion yuan with the CAGR of 24%. With the introduction of various guidelines and policies to encourage innovation, the number of new Chinese medicines declared for clinical trials has been increasing year by year in the past three years. 239 innovative Chinese medicines were successfully listed from 2001 to 2011. The R&D process of traditional Chinese medicine needs to go through the process of topic selection, IND, clinical trial, approval, NDA, production, etc., and the overall time-consuming process is relatively long, and some innovative medicines have taken more than 10 years from IND to market.