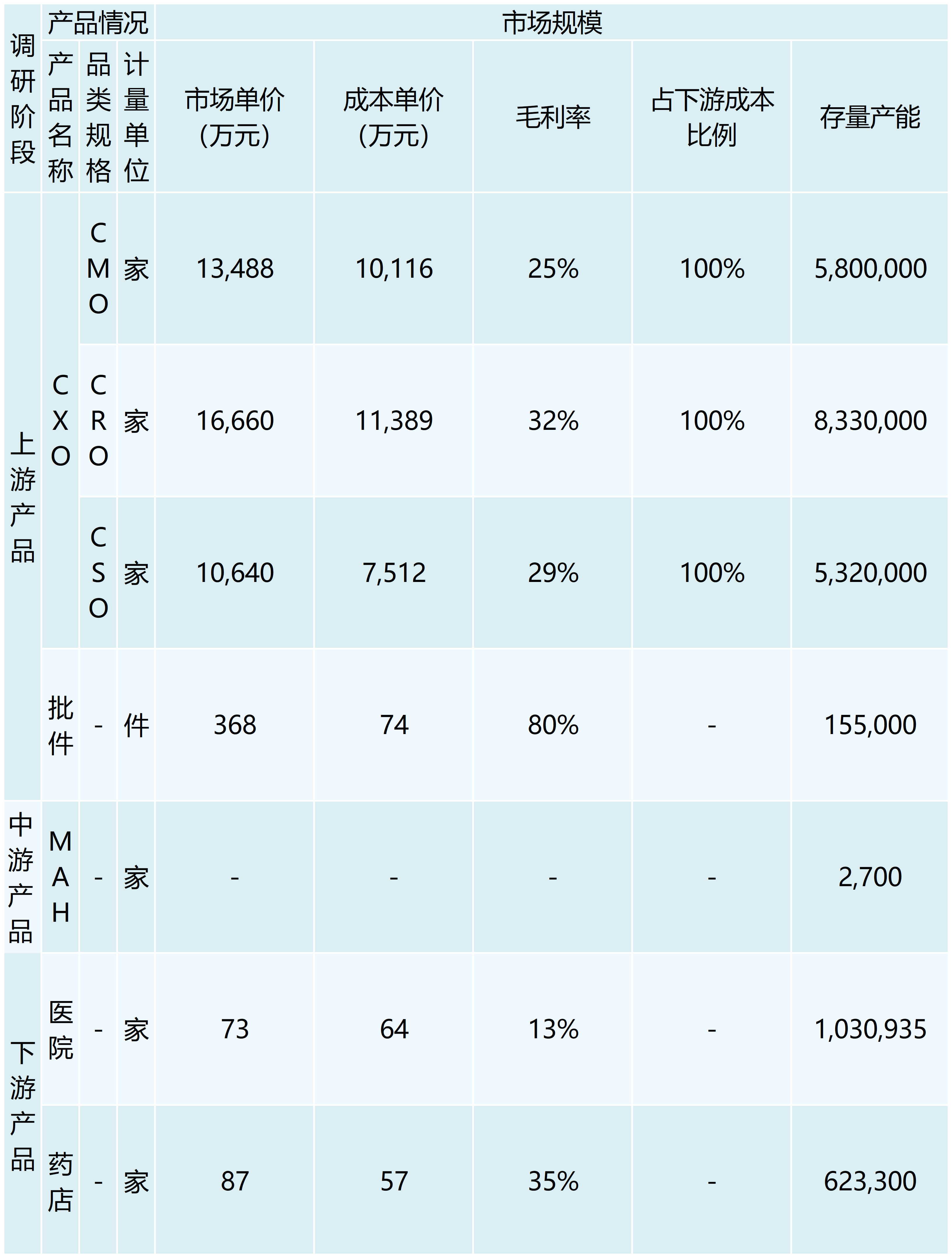
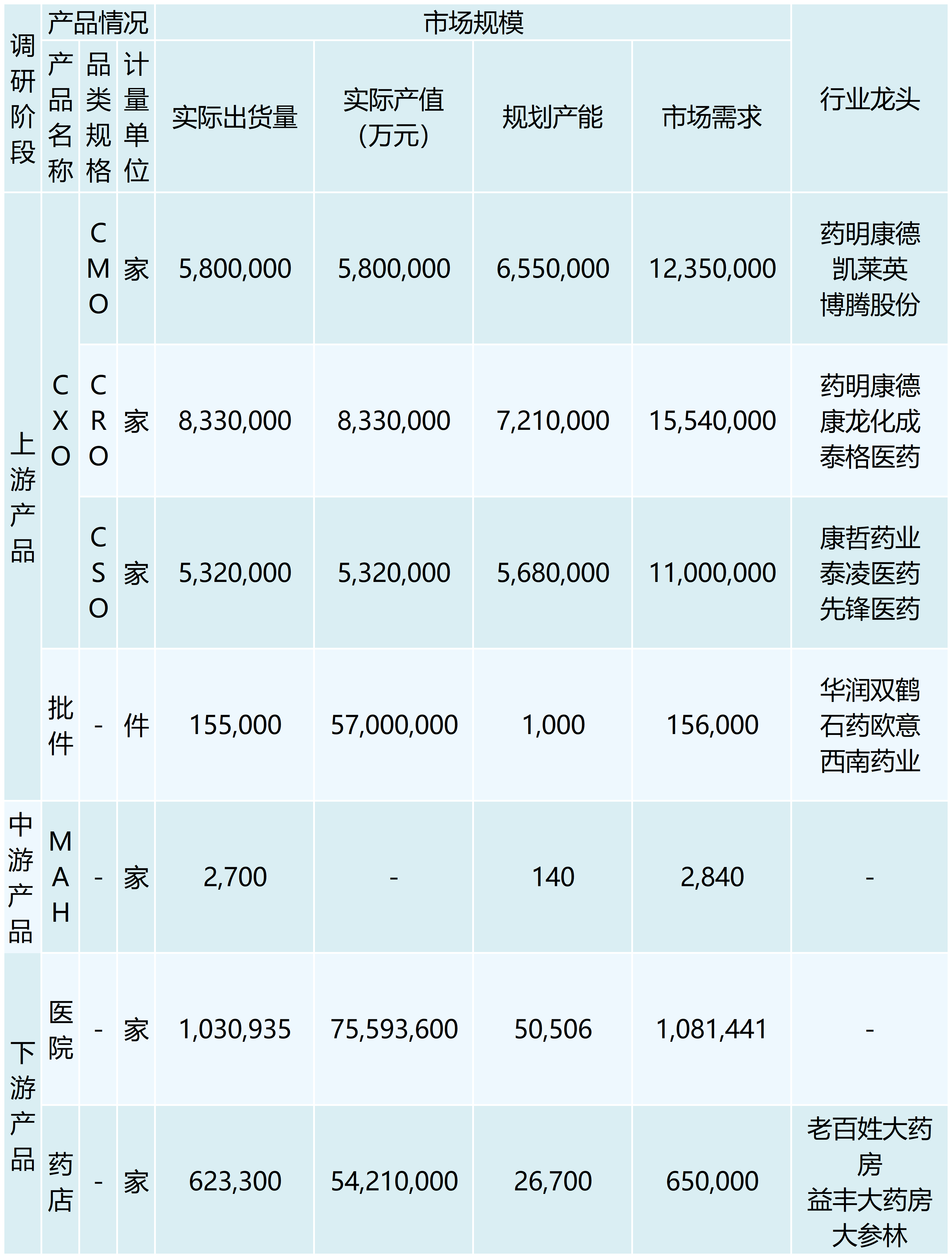
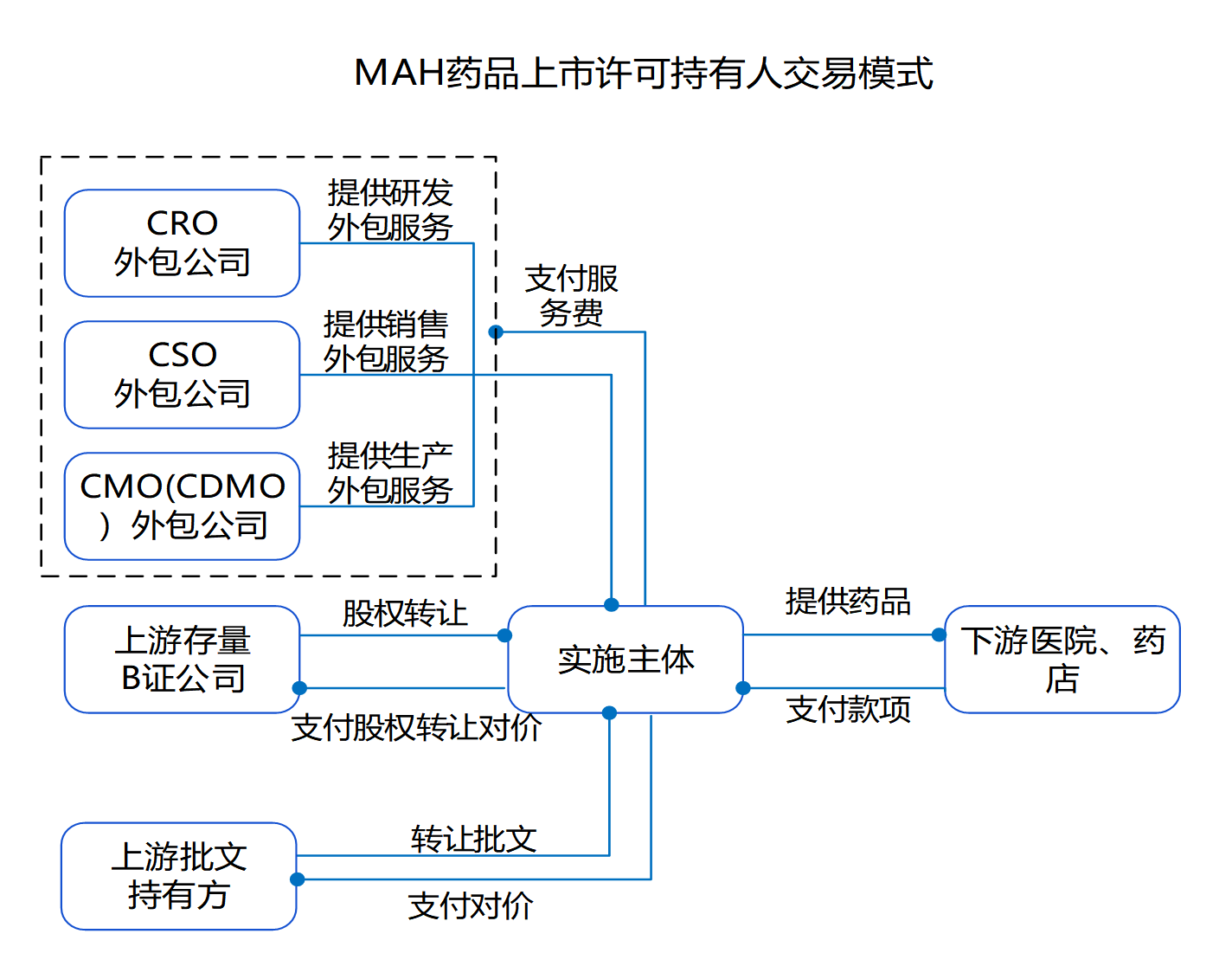
The Medicines Marketing Authorization Holder (MAH) system is a new system established after the revision of the Drug Administration Law was completed on December 1, 2019, which originated in Europe and the United States, and is a system model that manages the marketing authorization of medicines separately from the manufacturing authorization. It usually refers to a system in which drug research and development institutions, researchers, drug manufacturers and other subjects with pharmaceutical technology take primary responsibility for the quality of pharmaceuticals throughout their life cycle by filing an application for and obtaining a marketing authorization approval for a drug. According to data from the NCDC, the number of drug MAH subjects in China will be 2,440 in 2020, and the number of drug MAH subjects in China is expected to be around 2,700 by 2022, with an annual growth rate of 5.19%. By entrusting the authorization of other manufacturers to produce or sell operating companies to sell, rather than in the past will be bound to the approval of the production company, which will inevitably promote the pharmaceutical service outsourcing of the three forms of upstream hair R & D outsourcing CRO, production outsourcing CMO, marketing outsourcing CSO of the explosive growth. As of 2022, China's CXO market size reached 194.5 billion yuan, is expected to reach 38 million yuan in 2025, with an annual growth rate of more than 25%.